Bladder Issues for Spinal Cord Injury People
Learn more about the new clinical study EG110A-001-01!



What is the Study About?
EG110A Therapy
This will be a first study in any humans for EG110A – a specially designed and targeted investigational product.
EG110A is a designed new, non-multiplying gene therapy vector (i.e. transporter) delivering a study drug targeting specific nerve cells close to the spinal cord which control the bladder.
Proposed Therapeutic Action
This therapy is aimed at blocking specific sensory nerve signals from the bladder to reduce the triggers for involuntary bladder contractions thereby reducing bladder leakages.
Proposed Therapeutic Effect
The expectation is to improve continence for a long period of time, possibly over several years, after only one study treatment administration.




Who can join this study?
Participant Requirements
This study will be focused on people with neurogenic bladder, also called neurogenic detrusor overactivity, due to spinal cord injury.
We will be enrolling participants who are living with involuntary bladder leakages, also called urinary incontinence even under their current treatments.
Age
18-75 Years old
Gender
All genders
Spinal Cord Injury
The spinal cord injury needs to have been any level above the sacrum. Spinal cord injury must be at least 12 months post-injury before participating in this study.
Urinary Incontinence
Participants need to have chronic urinary incontinence (experience several urinary incontinence episodes per week) and perform daily clean intermittent catheterization.



How will the Drug be administered?
The study drug will be administered as a one-time treatment via multiple injections into the bladder wall. This is similar to the way botulinum toxin as BOTOX® (which is an approved treatment) is administered into the bladder.
Anesthesia
The study doctor will decide together with the participant which form of numbing/anesthesia will be the best.
Hospitalization
The participant will be asked to stay one night in the hospital after the study drug administration for observation.



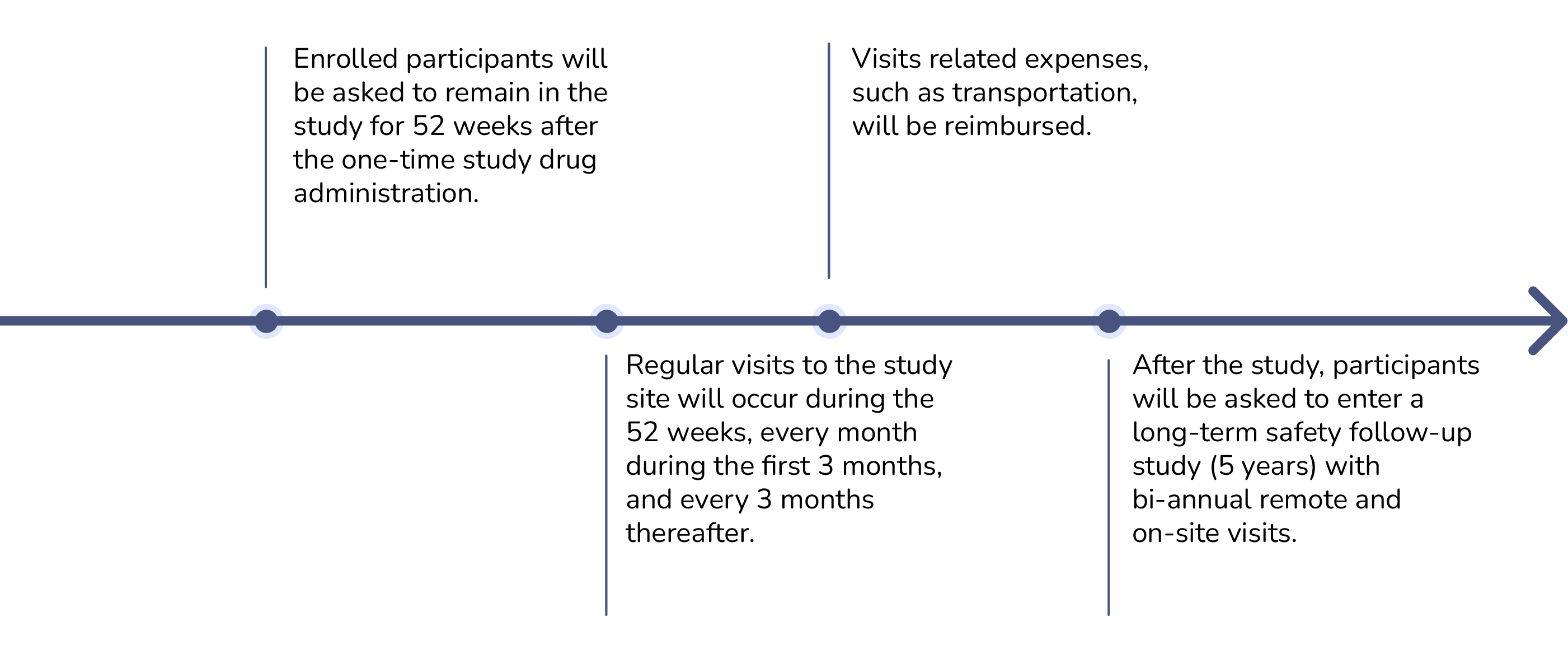
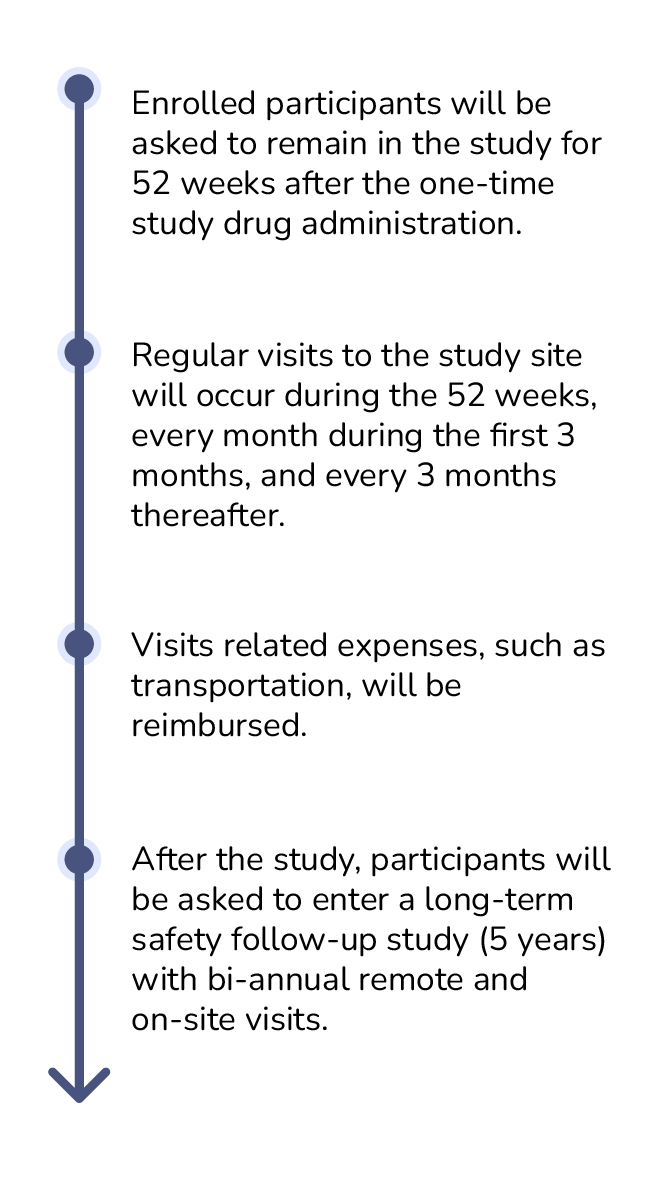
How long is the study?
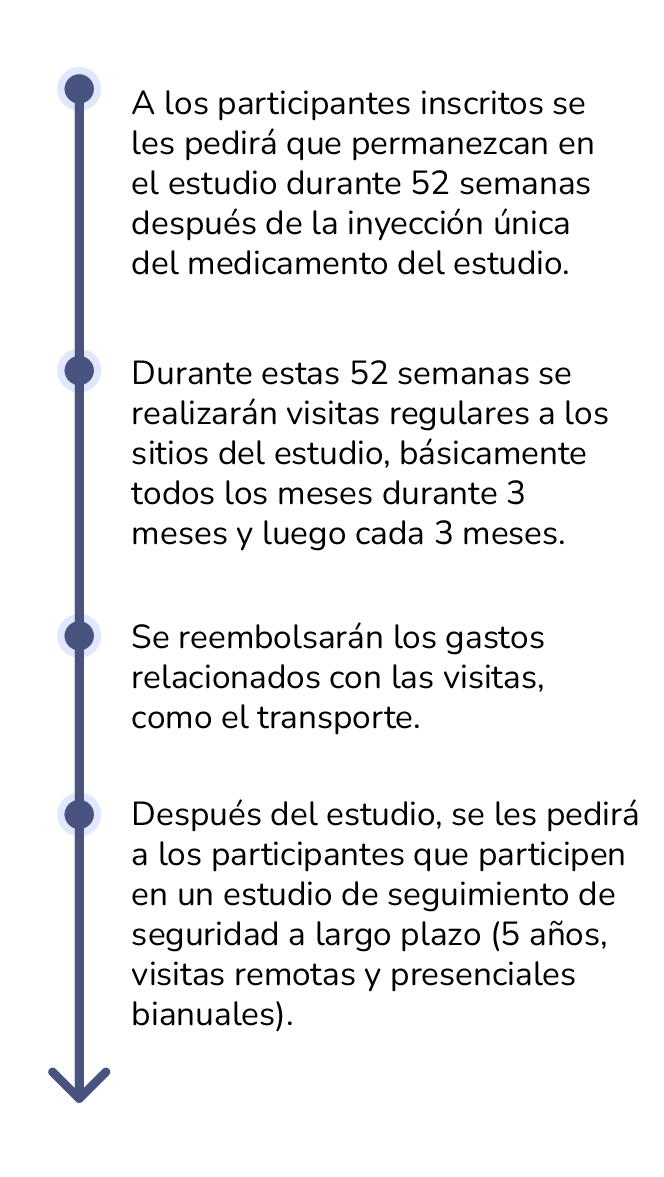
Finding Study Sites
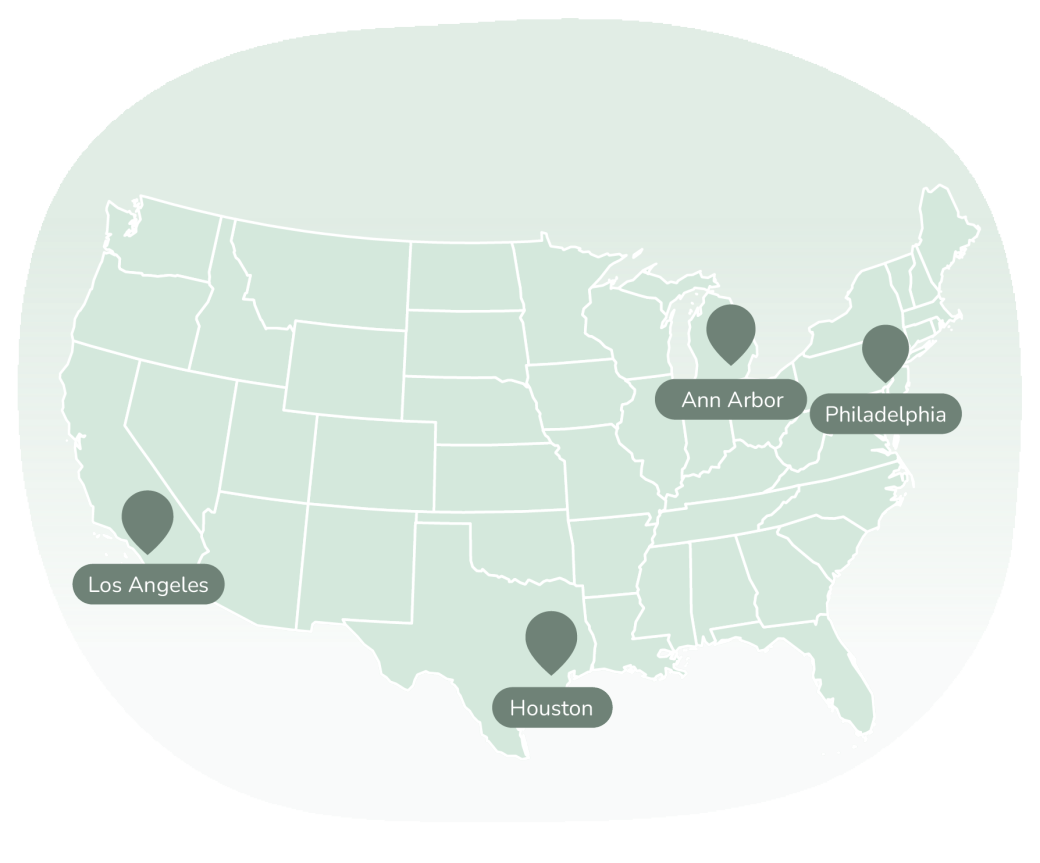
Please see the list of study sites and their location below with their contact information.
Feel free to contact the study sites directly if you are interested to learn more about this study!
For more information about clinical studies visit: www.clinicaltrials.gov